|
|
|
|
|
Oxygen |
|
|
|
Silicon |
|
|
|
Aluminium |
|
|
|
Iron |
|
|
|
Calcium |
|
|
|
Magnesium |
|
|
|
Sodium |
|
|
|
Potassium |
|
|
|
Titanium |
|
|
|
Hydrogen |
|
|
|
Manganese |
|
|
|
Phosphorus |
|
|
|
All others |
|
|
There are 12 elements that make up 99.23% of the earth's crust. Certainly all of the major minerals contain one or more of these elements. Of the 12, the first 8 combined with their volume make up the majority of the mass.
The more rare elements often substitute for the common ones in part of the crystal structure. In a few cases, the elements are so nonreactive that exist mainly as themselves and form very few mixed mineral structures. Ag (gold) and Pt (platinum) are in this category.
Since silicon and oxygen are first and second on the list it makes sense that they probably exist together in many minerals, and in fact there is a whole category of minerals know as silicates.
Minerals are studied in groups called classes. The standard Dana classes are listed in the table below. The classes are separated by the elements contained within each.
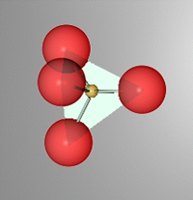
Silicates are the most abundant category of minerals in the earth's crust. The "silicate" ion is a very strongly covalent ion and is the most abundant mineral component in the crust. Silicate are the largest class of minerals.
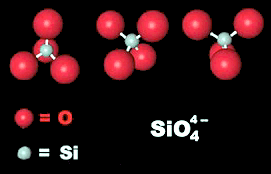
Other mineral categories include elements, pure atomic compounds. Oxides, those minerals containing O-2 or OH-1 (Hydroxides) but not SiO4-4. Carbonates, CO3-2 , Sulfates SO4-2 , Sulfides S-2 , Halides (F- , Cl-, Br- ...), Borates BO3-2, and Nitrates NO3-. The book also mentions.
Because the silicate class is so large and abundant, it is further broken down in to subcategories based on the silicate bonding structures.
|
Mineral Classes |
|
Elements |
|
Silicates |
|
Oxides |
|
Carbonates |
|
Sulfides |
|
Sulfates |
|
Phosphates |
|
Tungstates/Molybdates |
|
Halides |
|
Borates |
|
Nitrates |
 |
 |
|
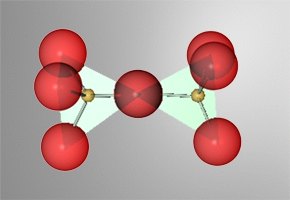
We have seen a simple silicate anion SiO4-4, but it is possible to put two such anions together and have them share one oxygen. The resultant structure is a Si2O7-6 species. Since the shared oxygen is electron satisfied, the charge drops from the expected (-8) for two silicate single ions, to (-6) for the complex cation formed. The process of combining like units together is called polymerization. |
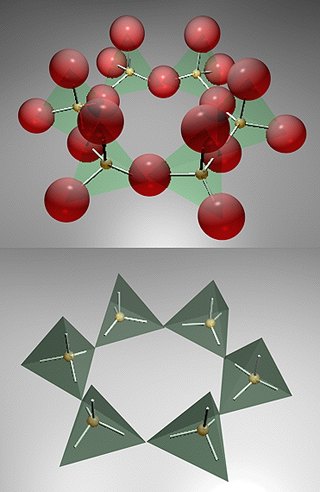
The silicate cation can combine to form an array of structures. The structures can be linear (in a straight line), circular (ring like), or can be three dimensional.
The silicate minerals are differentiated by the type of polymerization that the silicon adopts. Silicates can even form ring structures like the one illustrated. This would be a structure with the following formula ...
Si6O18-12
| NEXT | TOC | PREV |